Click the reaction arrows in sequence to view the 3D models and animations respectively
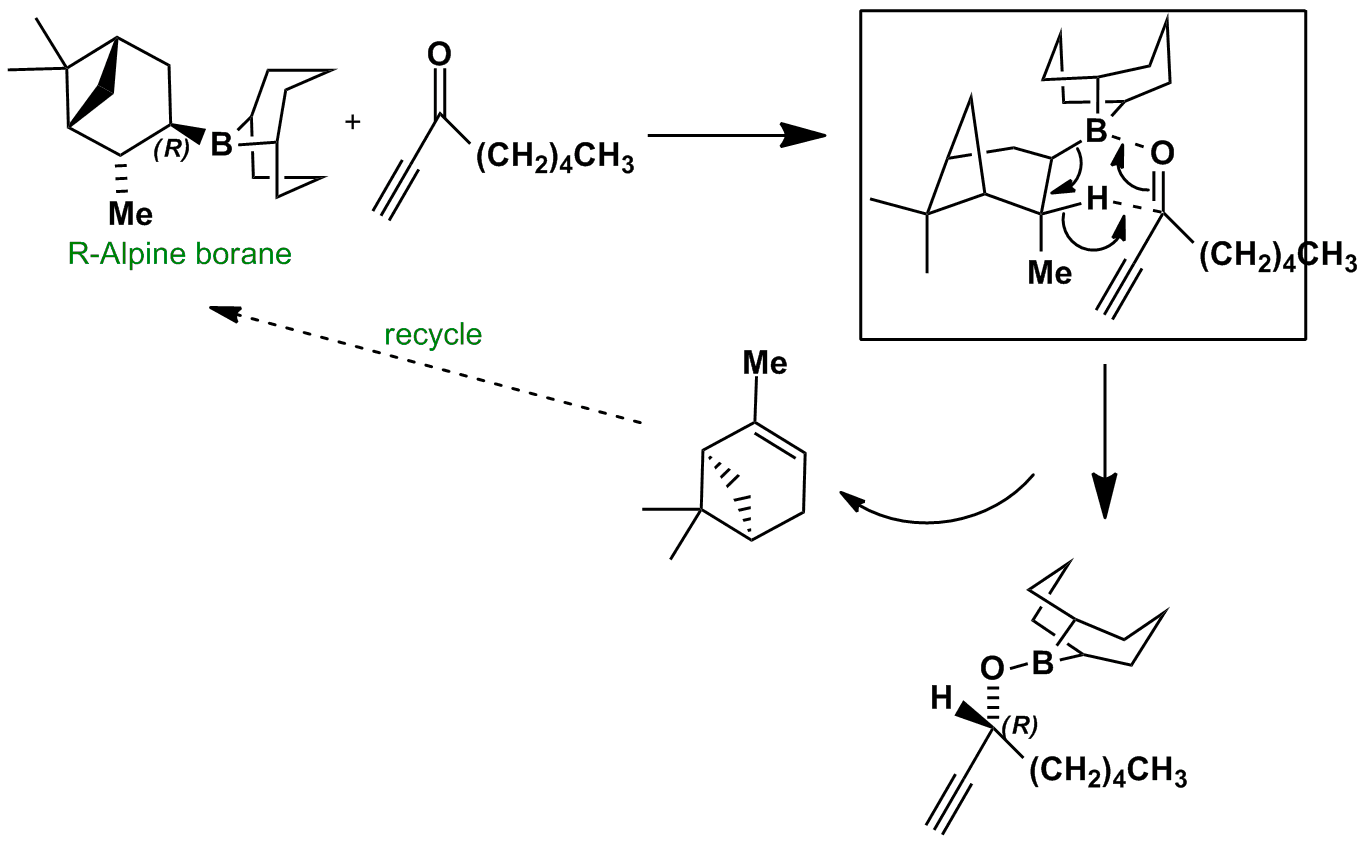
R-Alpine borane is effective at reducing acetylenic ketones to secondary alcohols with reasonable enantioselectivity. The enantioselection arises through the selective placement of the sterically undemanding alkyne close to the methyl substituent in the boat transition state for the reduction.
M. M. Midland, Chem. Rev., 1989, 89, 1553–1561.