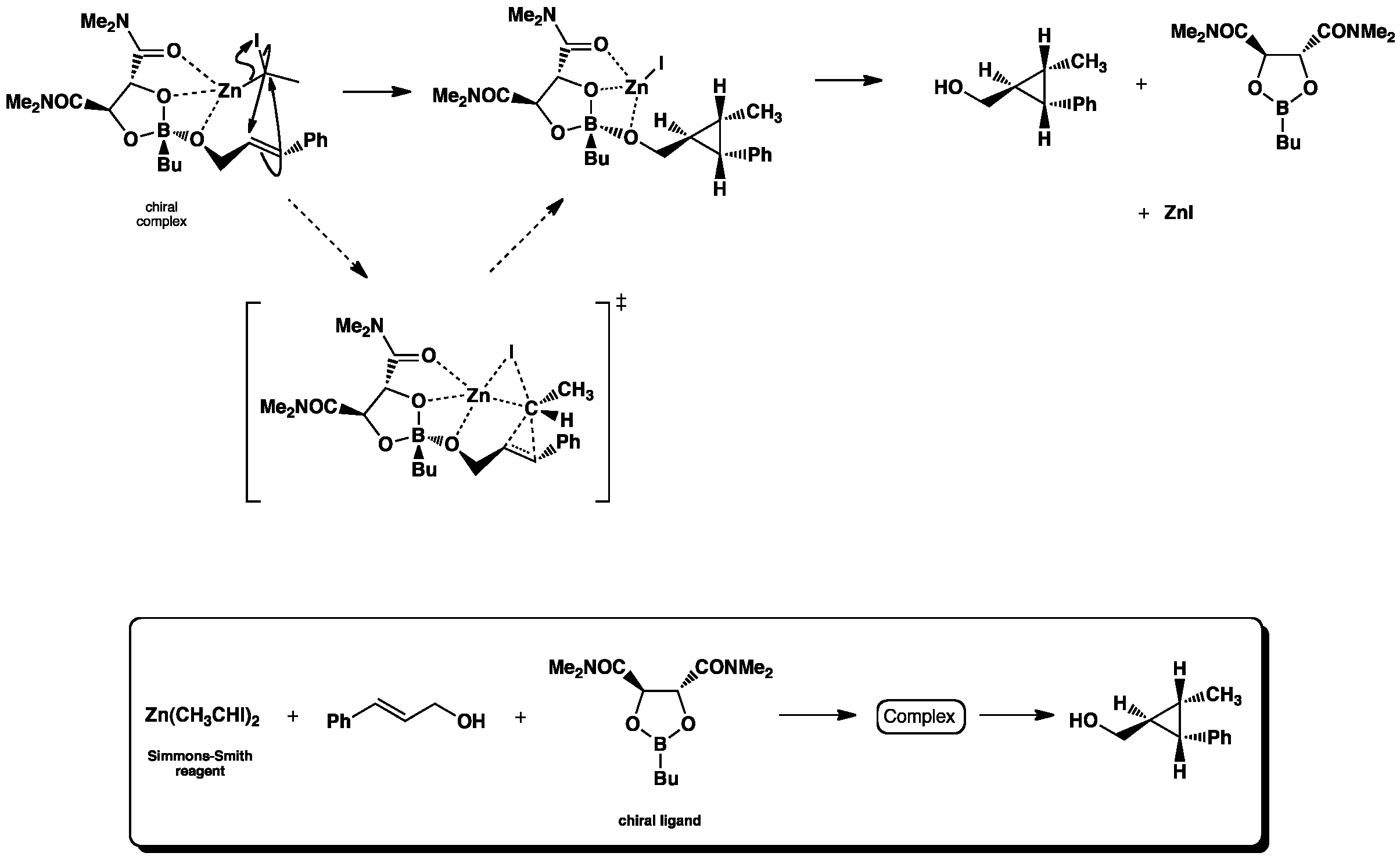
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The asymmetric Simmons-Smith reaction is a cyclopropanation where a carbenoid (intermediate zinc-iodine complex) reacts with an alkene to from a stereospecific cyclopropane via a cheletropic reaction. The oxygen atoms of the dioxaborolane ligand, along with the hydroxyl group of the alkene reagent, coordinate with the Zn atom anchoring the complex into a postion where only one stereoisomer can be formed.
T. Wang, Y. Liang and Z.-X. Yu, J. Am. Chem. Soc., 2011, 133, 9343–9353.