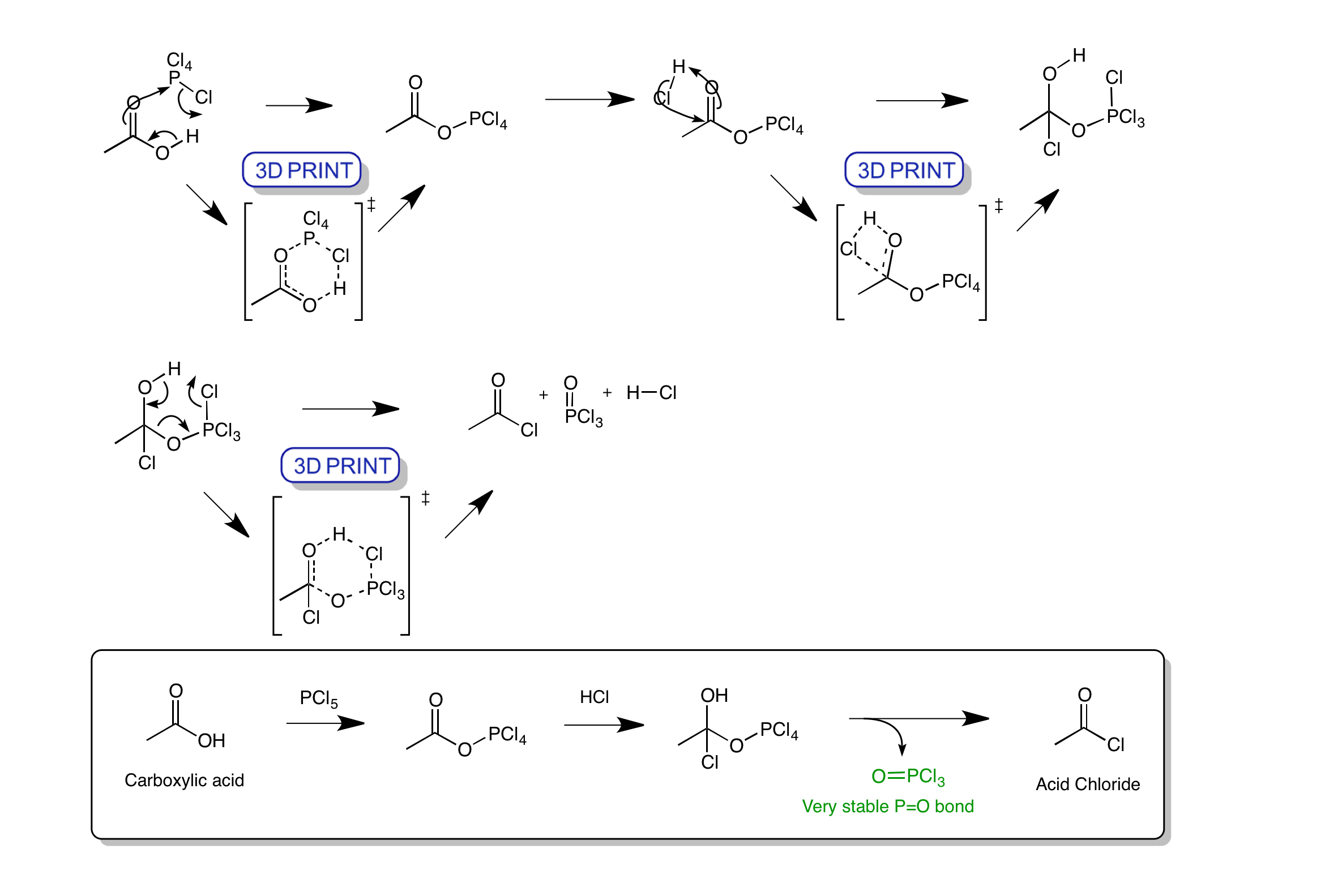
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The reaction of a carboxylic acid with phosphorus pentachloride produces an acid chloride. The reaction goes firstly by a cyclic transition state with the removal of HCl. Then via a nucleophilic addition of chloride where the carbonyl is simultaneously protonated. The final step is a cyclic transition state to form the acid chloride, HCl and phosphorus oxychloride. Formation of the strong P=O bond drives the reaction.
M. Green and D. M. Thorp, J. Chem. Soc. B Phys. Org., 1967, 0, 1067.