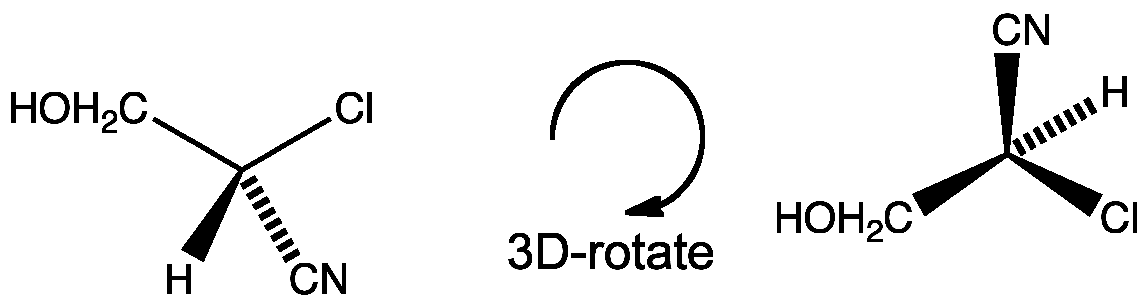
Click the structures and reaction arrows to view the 3D models and animations respectively
Consider this molecule. Assign the priorities, rotate into the correct orientation and decide if it is R or S.
Step 1 | Assign the atoms numbers 1 to 4 based on highest atomic number. In this example chlorine has the highest atomic number and is assigned 1 whereas hydrogen has the lowest atomic number and is assigned 4 |
Step 2 | Arrange the molecule so that the lowest priority substituent faces backwards in this case number 4 |
Step 3 | Mentally move from substituent 1 to 2 to 3. If you are moving clockwise assign the label R. If anticlockwise assign the label S |
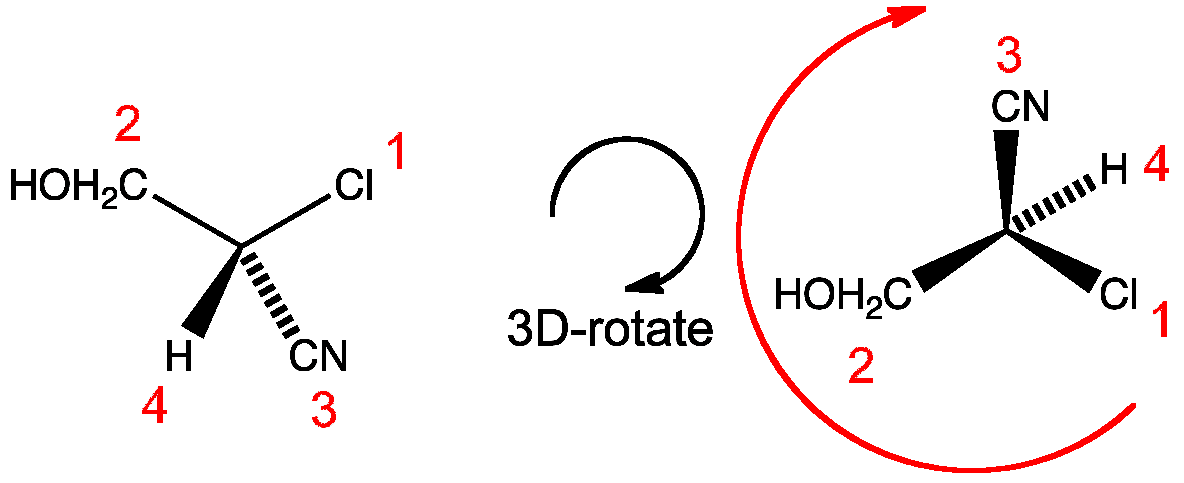
R as clockwise