Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
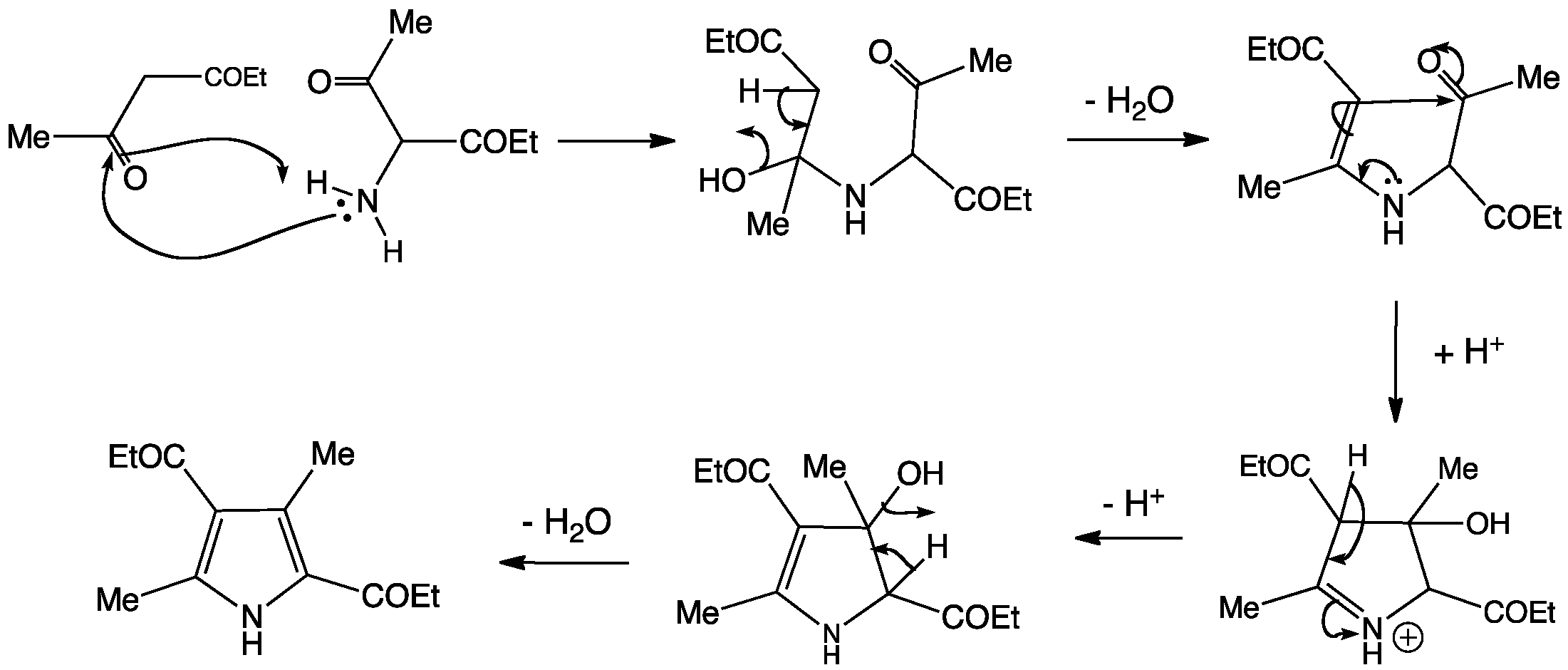
This synthesis is so important, it was given the name of its inventor, Ludwig Knorr.
In this reaction, the amino group forms an imine with one of the diketone carbonyl groups. The enamine intermediate then cyclizes onto the other ketone carbonyl and water is removed to form the aromatic heterocycle.
A. R. Katritzky, D. L. Ostercamp and T. I. Yousaf, Tetrahedron, 1987, 43, 5171–5186.