Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
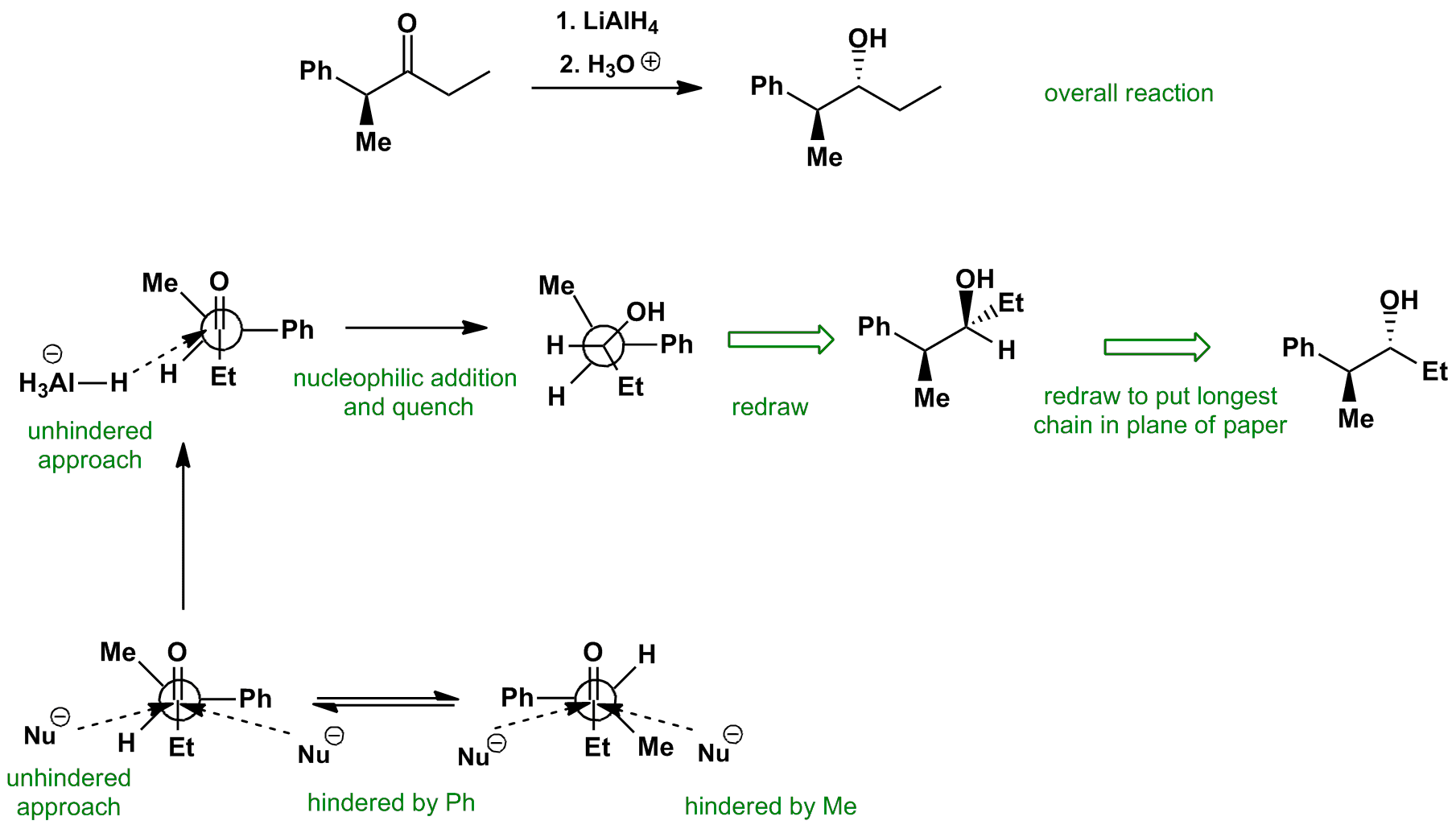
The Felkin-Anh conformation has the largest (and most electronegative in other cases) group perpendicular to the carbonyl. Attack on the Bürgi-Dunitz trajectory leads to the nucleophile, hydride in this case, passing close the substituents on the alpha carbon. The nucleophile, in this case, hydride, attacks from the least hindered approach.