Click the reaction arrow in sequence to view the 3D models and animations respectively
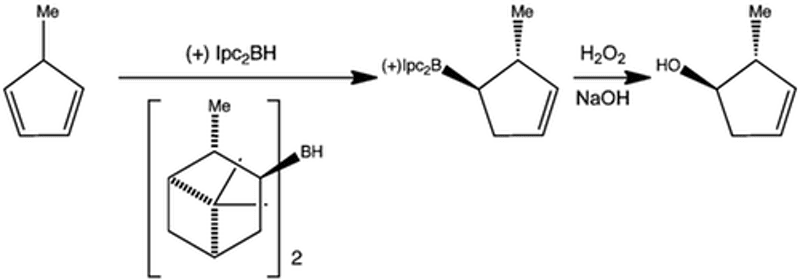
Diisopinocampheylborane (Ipc)2BH (from pinene) is an enantioselective hydroboration reagent. In the case of a substituted cyclopentadiene, attack occurs opposite the methyl group to establish two stereogenic centres. The two terpene fragments dictate the enantioselectivity. The methyl group can only accommodate a hydrogen from the alkene in the transition state.
The product borane is converted to the alcohol with retention of stereochemistry by the H2O2/NaOH mechanism.
H. C. Brown and P. Veeraraghavan Ramachandran, J. Organomet. Chem., 1995, 500, 1–19.