Stereoselective Wittig Reaction-Overview
‘Click’ the different stages to view 3D models of the Z- and E-stereoselective examples of the Wittig reaction mechanism:
Z-Selective Wittig Reaction
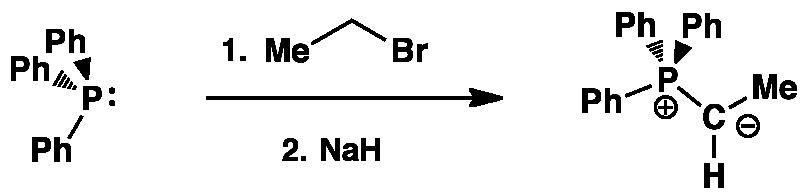
Stage 1:Unstabilised Ylid Formation
The nature of the selectivity of the Wittig reaction is chosen by the type of phosphonium ylid used. The Z-selective reaction is favoured with an unstabilised phosphonium ylid as in the example below.