Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
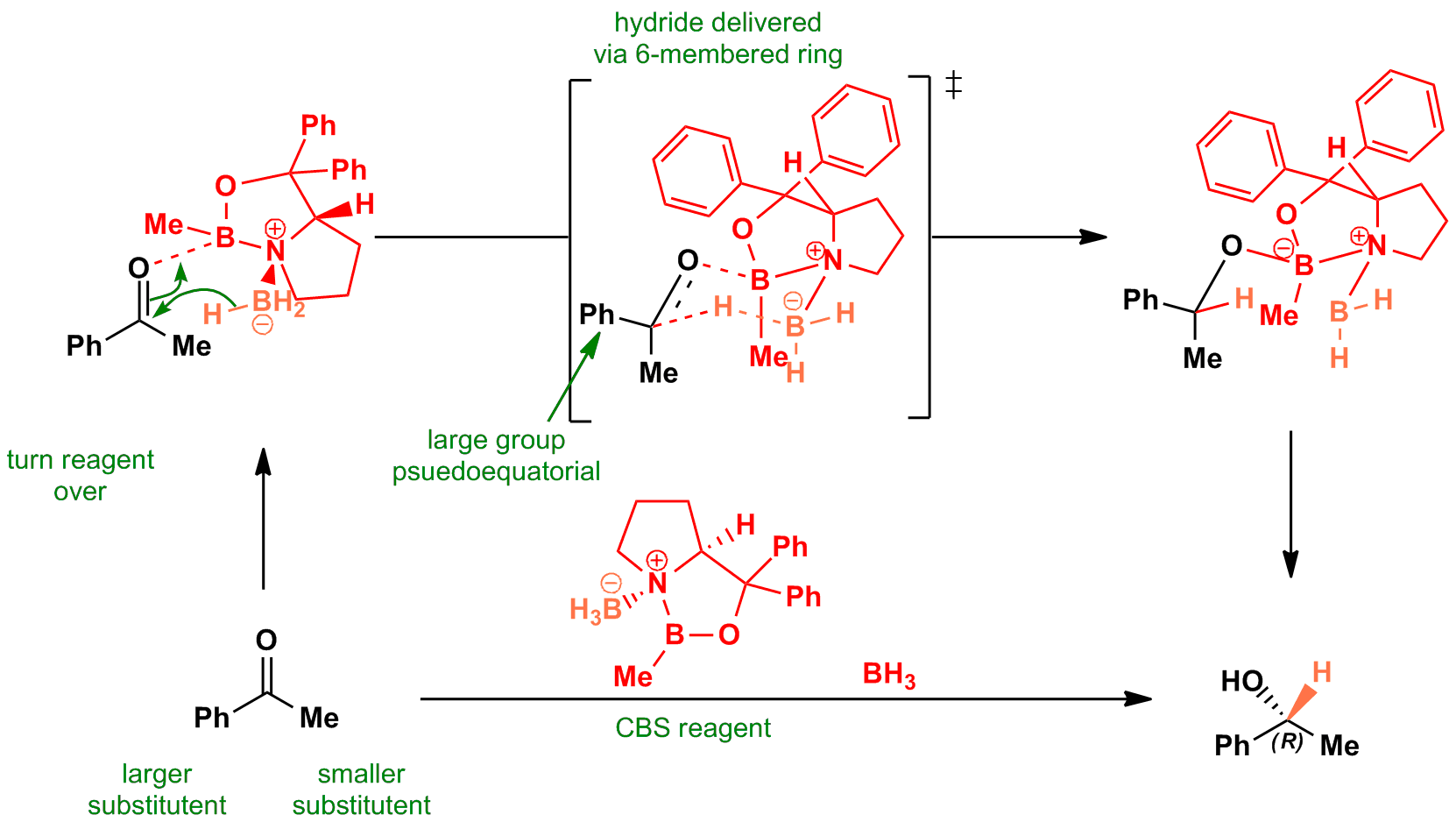
This reduction works best when the two carbonyl substituents are well-differentiated sterically – ie Ph and Me. When the carbonyl oxygen is complexed to the boron it becomes electrophilic enough to be reduced by the weak hydride source. The hydride itself is delivered via a six-membered cyclic transition state. The enantioselectivity arises from the preference of the larger carbonyl substitutent for the pseudoequatorial position of the ring.
J. Vargas-Caporali and E. Juaristi, Synthesis (Stuttg)., 2016, 48, 3890–3906.