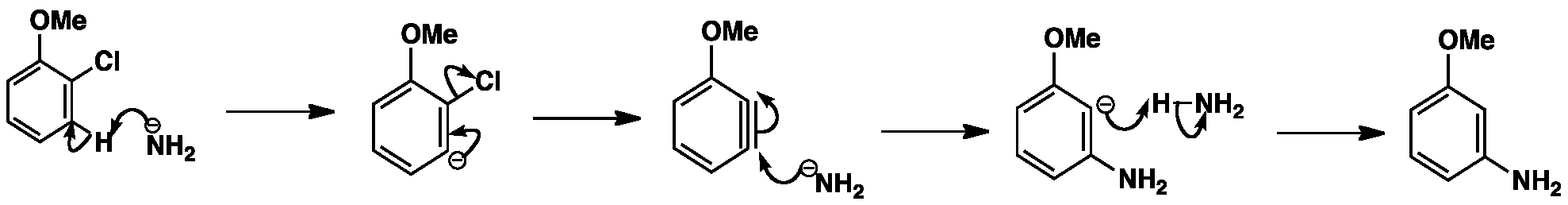
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The reaction begins with the removal of a proton ortho to the leaving group by a strong base e.g. sodium amide, sodamide. The next step is the loss of the leaving group in an elimination reaction resulting in a benzyne intermediate. Benzyne is a very unstable intermediate that is attacked by nucleophiles followed by protonation to give the final product. The benzyne intermediate can be attacked at either end so the characteristic loss of regiochemistry in the substitution can result.
H. H. Wenk, M. Winkler and W. Sander, Angew. Chemie Int. Ed., 2003, 42, 502–528.
P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550–3577.