Each atomic orbital page contains some information about the type of orbital with an interactive Jmol window providing a 3D visualisation of their shape.
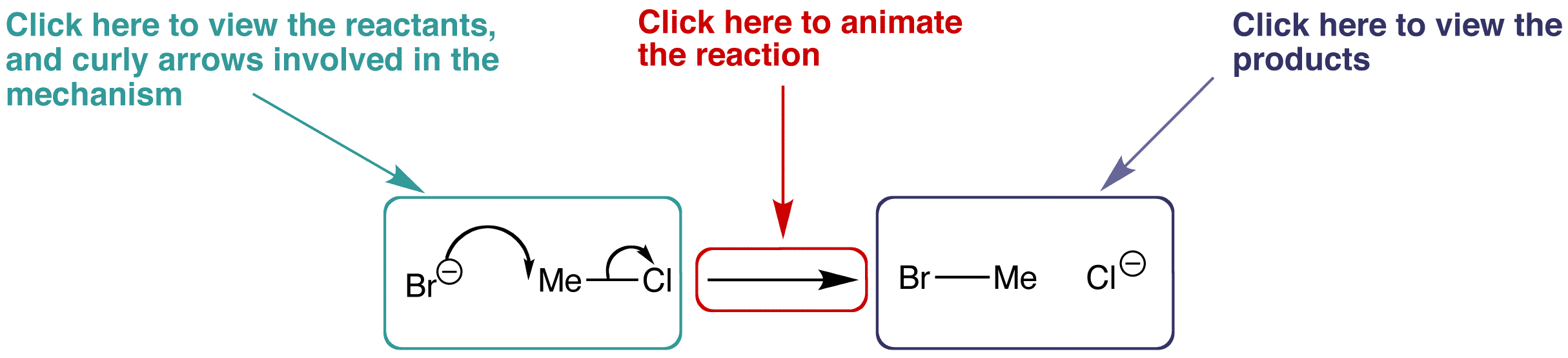
The organic mechanism pages contain some information about the reaction, followed by an interactive reaction scheme similar to the one below.
By clicking on the appropriate parts of the scheme and rotating the structures in 3D, you can explore the characteristics of the reaction and discover how the molecules interact with one another.
The crystal structure and polymer pages show selected examples relevant to A level, you will find many more in the Inorganic and Polymer sections respectively.
There is an introduction to proton NMR showing protons in equivelent environments and an explanation of the shapes of molecules based on electron counting (VSEPR).
Finally you can create and explore your own molecules in the Molecular Playground.
Use the example below to familiarise yourself with the layout of the organic mechanism pages.
Use your scroll wheel to enlarge and shrink the models, right clicking the mouse (Control-click MacOS) over the resizeable Jmol screen will give additional options and features that can be explored for the reaction.
To navigate your way to the required page, simply use the side-bar. Select the topic you are looking for and then choose from the given list.
Additional buttons below the Jmol applet are available and perform the labelled features.