Di-2-ethyl hexyl phosphoric acid (D2EHPA) is used as a ligand to extract metal ions such as zinc from acidic aqueous solutions into hydrocarbon solvents. D2EHPA has a very polar phosphoric acid “head” group and non-polar hydrocarbon “tail” groups. The electrostatic surface shows the polar regions (red and blue) and the non-polar regions (green).
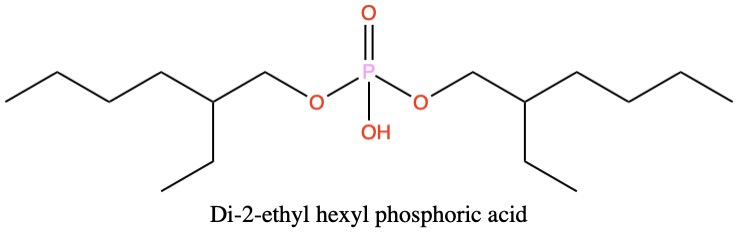
In the animation two molecules of D2EHPA solvate a zinc ion (Zn2+) to make a complex which is soluble in hydrocarbons such as kerosene due to the hydrophobic alkane chains surrounding the dication. The zinc ions are thus extracted from the aqueous layer to the organic layer and can be separated.