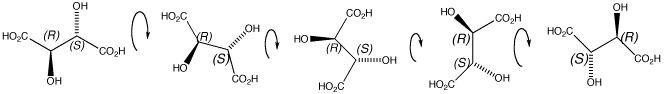
Click the structures and arrows to view the 3D models and animations respectively
The RS stereoisomer is exactly the same as the SR stereoisomer in this case because of the symmetry of tartaric acid. The mirror images are NOT enantiomers.
Prove this to yourself by clicking on the 2D structure on the left of the page, then on the arrows to see how the 3D molecule rotates to make it superimposable on the right hand 3D structure. Thus this compound is not chiral. It is in fact achiral.
Compounds that contain more than one stereogenic centre but are also achiral are described as meso compounds.