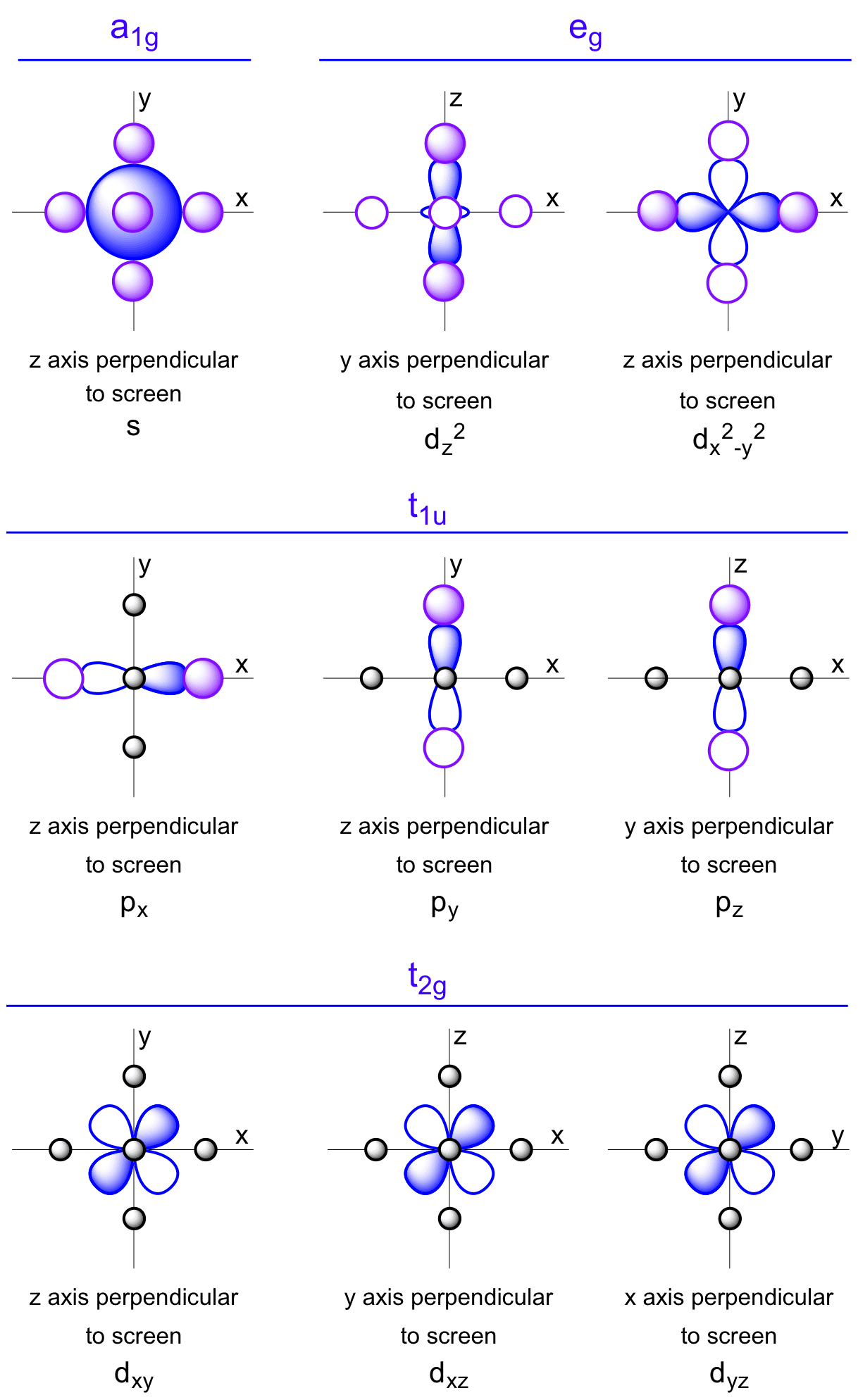
This series of diagrams illustrate the symmetry adapted linear combinations of ligand σ orbitals, which are represented by spheres.
The metal s orbital has a1g symmetry as it is symmetric to all operations. Hence it bonds with all the ligand σ orbitals in phase.
The p orbitals have t1u symmetry. The linear nature of p orbitals mean they only bond when pointing directly at ligand σ orbitals.
The d orbitals which have eg symmetry bond with ligand σ orbitals as they point directly at them.
The d orbitals which have t2g symmetry are non-bonding with ligand σ orbitals, as the ligand σ orbitals see equal amounts of in phase and out of phase orbital, hence a net zero interaction.
Orbital-orbital overlap and SALC Homepage