NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
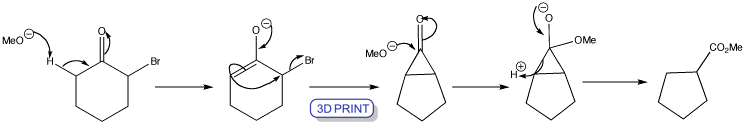
Favorskii rearrangement of cyclic 2-bromoketones leads to a ring contraction. Enolization occurs on the side of the ketone away from the bromine atom and the enolate cyclizes. The cyclopropanone intermediate is symmetrical so that the product is the same whichever C–C bond breaks after nucleophilic attack by the methoxide ion.