
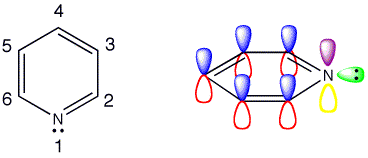
Click the checkboxes to view the atom numbering and p orbitals respectively
Pyridine is formed by replacing one of the CH groups in benzene with a nitrogen atom. The orbitals in the ring have not changed significantly and there are still the six pi-electrons from the three double bonds in the aromatic structure. Instead of the CH bond in benzene the nitrogen has a lone pair of electrons to occupy this space. Pyridine is an imine, which are usually unstable, but due to the aromaticity pyridine is a stable imine.
Reactions with nucleophiles take place preferentially at the 2-, 4- and 6- positions due to the nitrogen atom lowering the energy of the LUMO. By looking at the LUMO you can see that the lobes are bigger at these positions, which means they are preferred positions for nucleophilic attack.