Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
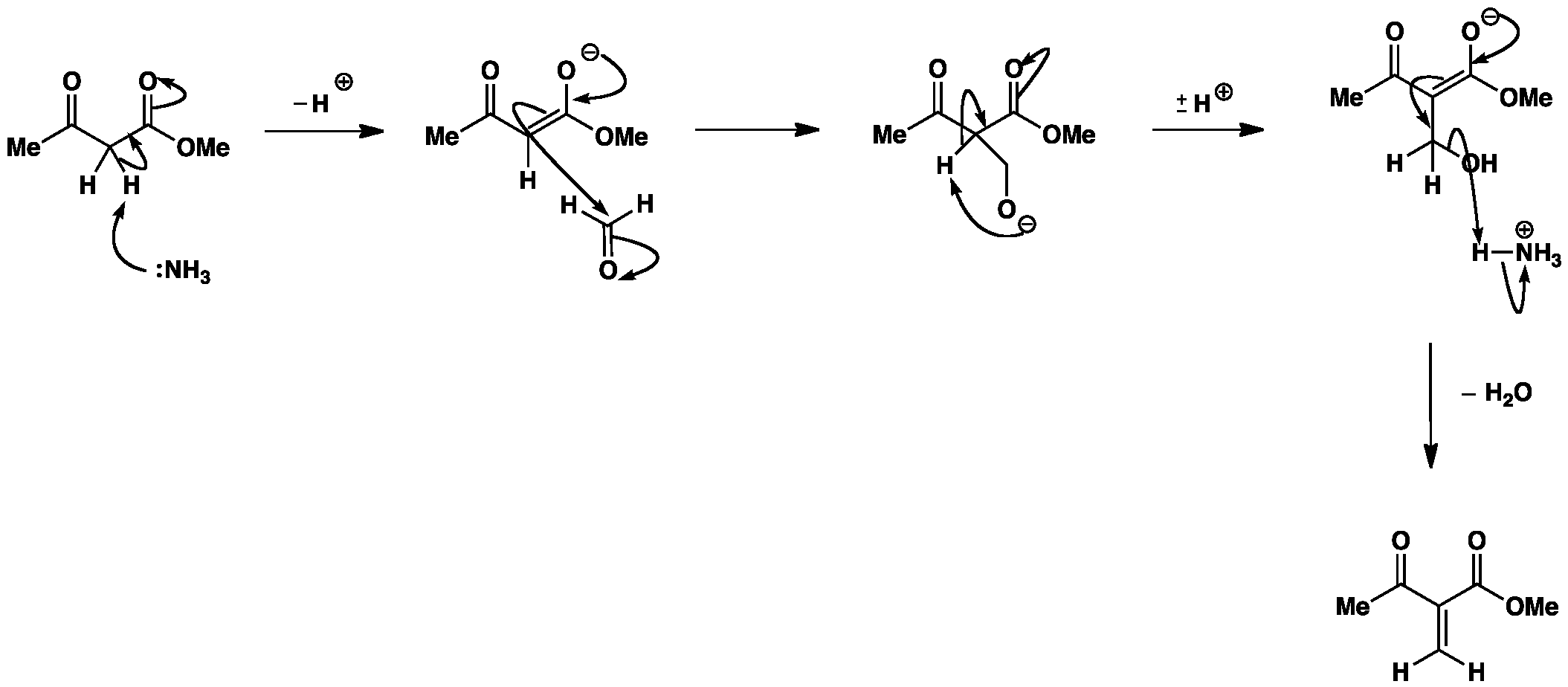
The first step of the reaction is the attack of the β-ketoester enolate on the aldehyde, followed by an E1cB elimination to give an unsaturated carbonyl compound.
Note: Step 1 and Step 2 can happen in either order
H. G. O. Alvim, E. N. da Silva Júnior and B. A. D. Neto, RSC Adv., 2014, 4, 54282–54299.