Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
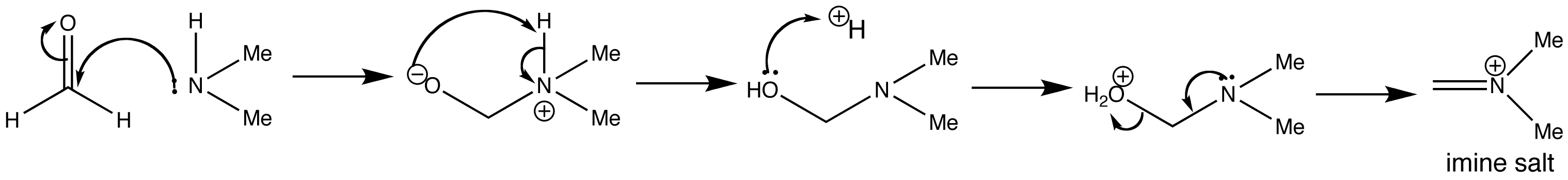
The first stage of the Mannich reaction is the formation of the iminium salt from the amine and formaldehyde. The amine is nucleophilic and attacks the more electrophilic carbonyl group of formaldehyde (relative to the cyclohexanone). No acid is needed for this addition step, but acid-catalysed dehydration of the addition product gives the imine salt, which is actually quite stable. The corresponding iodide is sold as ‘Eschenmoser’s salt’ for use in Mannich reactions.
T. F. Cummings and J. R. Shelton, J. Org. Chem, 1960, 25, 419–423.
H. G. O. Alvim, E. N. da Silva Júnior and B. A. D. Neto, RSC Adv., 2014, 4, 54282–54299.