Step 2 – Acid catalysed aldol addition
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
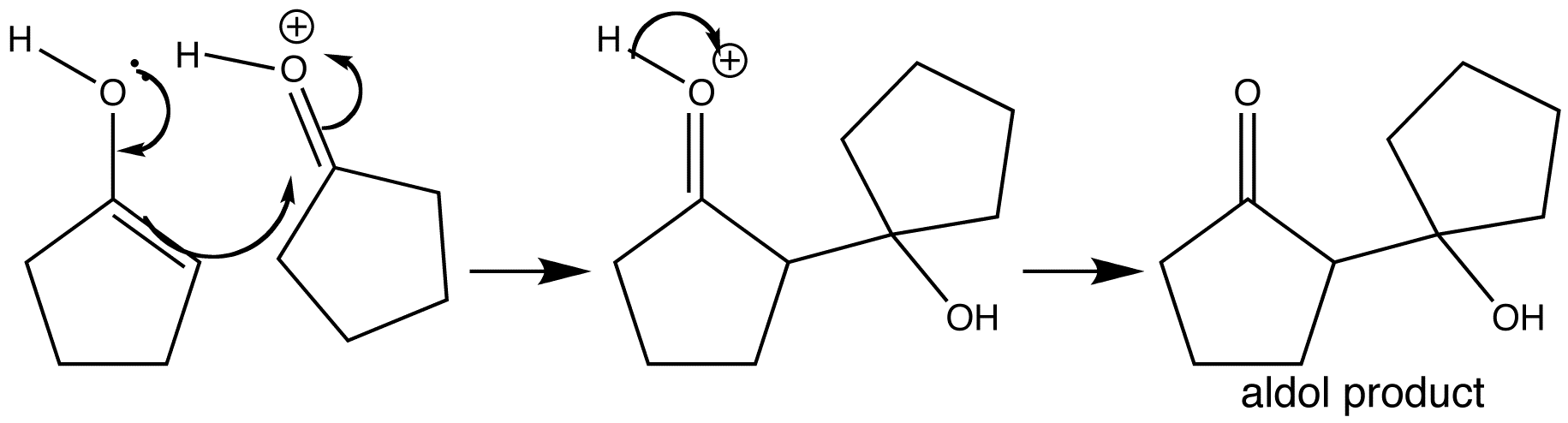
Enols are less nucleophilic than enolates, but this reaction occurs because the electrophilic carbonyl component is protonated. Following deprotonation of the carbonyl oxygen, the aldol product is formed, which in this case is a tertiary alcohol.
Back to cyclopentanone aldol summary
D. S. Noyce and W. A. Pryor, J. Am. Chem. Soc., 1955, 77, 1397–1401.
C. L. Perrin and K.-L. Chang, J. Org. Chem., 2016, 81, 5631–5635.