Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
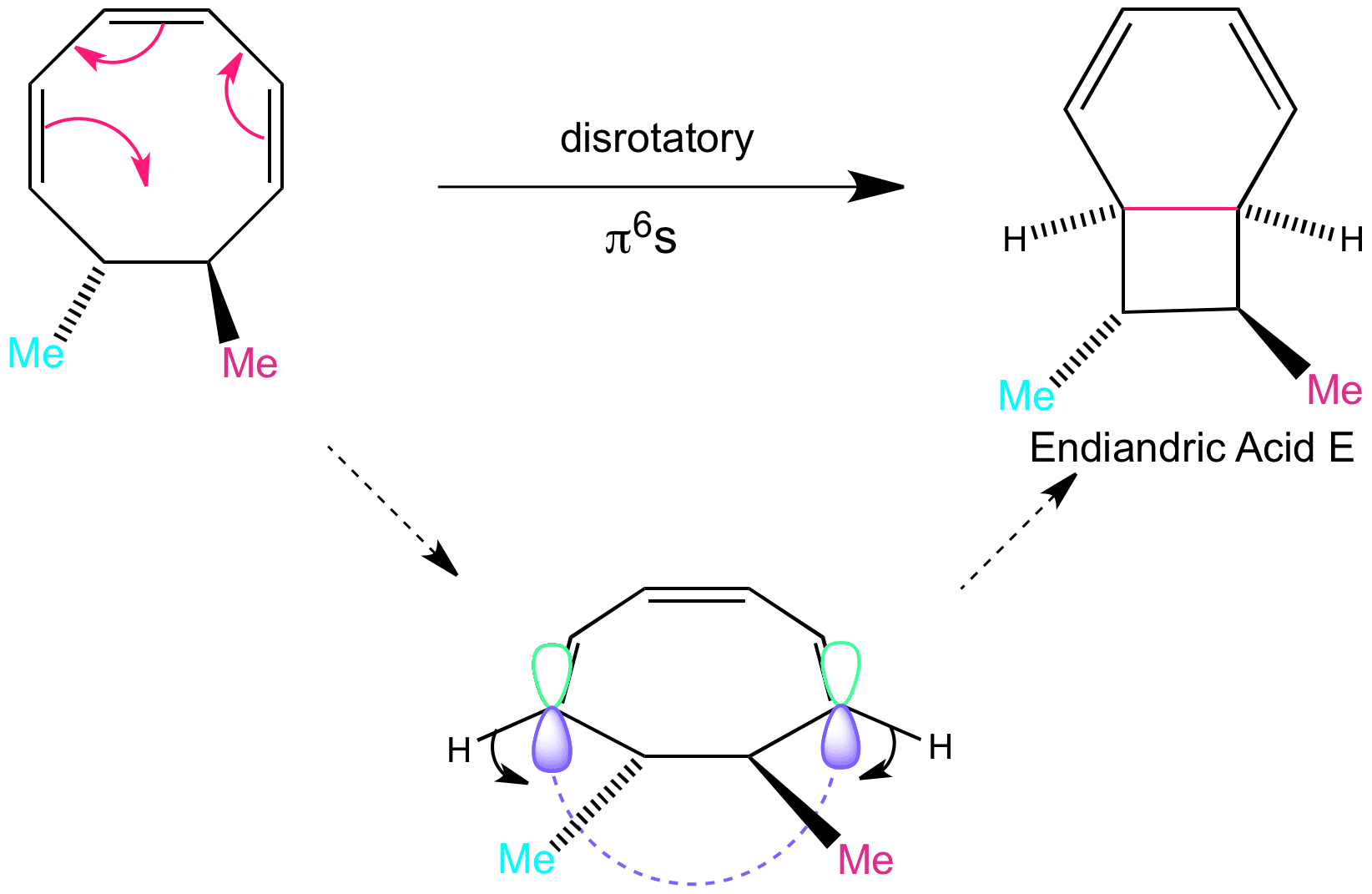
This is another direction of the disrotatory motion that can happen, which occurs on the bottom face. this results in the formation of endiandric acid E, the orbitals move “outwardly”. The hydrogens end up pointing downwards. Endiandric acid E can go on to do a further step in the tandem sequence which is again intramolecular and forms Endiandric acid A.
Please note that here the substituents that end up on opposite sides are modelled by methyl groups here. The berry coloured methyl is the allyl chain with a phenyl at the end, and the cyan coloured methyl is the carboxylic acid substituent.
Clicking on the word “disrotatory” shows the movement of the orbitals as part of the animation of the reaction. This animation is slow to load.
Final Year Project 2013: Ilona Blee
Other Option in Tandem – disrotatory to form Endiandric Acid D
K. C. Nicolaou, N. A. Petasis, R. E. Zipkin and J. Uenishi, J. Am. Chem. Soc., 1982, 104, 5555–5557.