Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
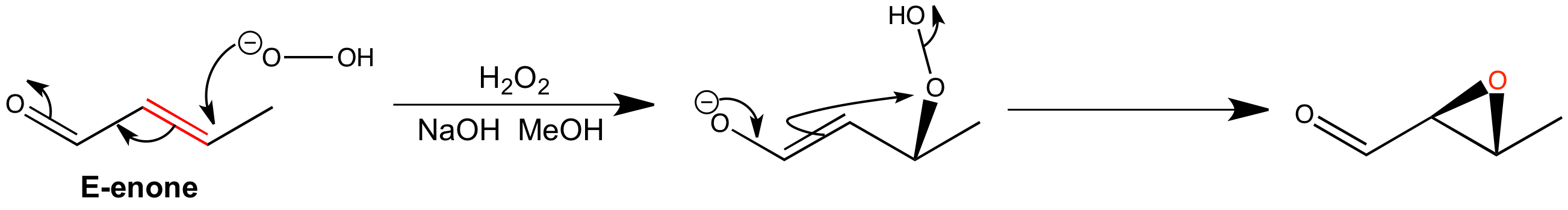
Epoxidation of the E-enone is relatively quick to complete and the epoxide is isolated in a high yield. The mechanism involves conjugate addition and ring closure with cleavage of the weak O-O bond. The closure of the three-membered ring is fast enough to preserve the stereochemistry of the intermediate enolate.
T. Hashimoto and K. Maruoka, Chem. Rev., 2007, 107, 5656–5682.