Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
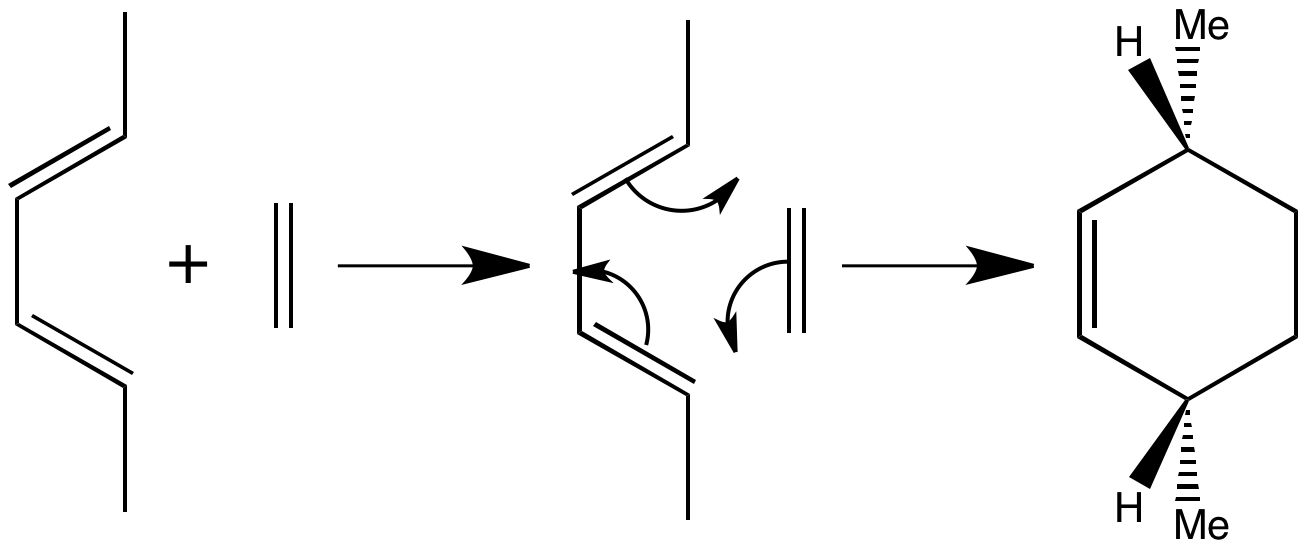
The two CH3 groups appear beneath the new six-membered ring, with the hydrogens above. There is a plane of symmetry throughout and the products must have this symmetry too, because the reaction is concerted and no significant movement of substituents can occur.
J. Sauer, Angew. Chemie Int. Ed. English, 1967, 6, 16–33.