Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
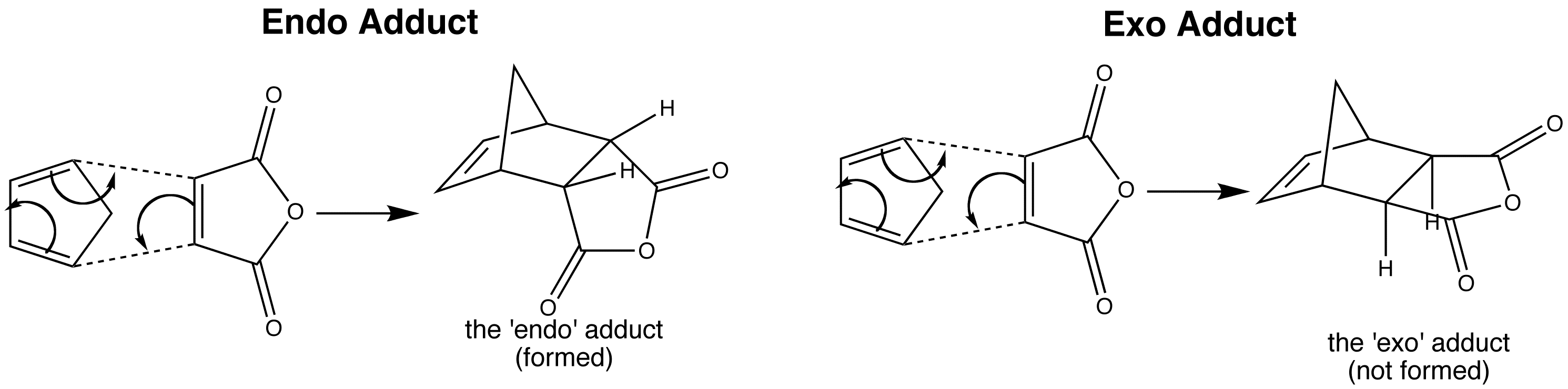
The Diels-Alder reaction between cyclopentadiene and maleic anhydride can produce two possible products, the ‘endo’ and the ‘exo’ adducts. This is because although the hydrogens of the maleic anhydride must be cis in the product, there are two possible arrangements where this is true.
The actual product formed is the ‘endo’ adduct. This diastereoisomer is the less stable one, but is formed because it is the kinetic product. There is also a bonding interaction between the carbonyl groups of the dienophile, and the developing π bond at the back of the diene.