Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
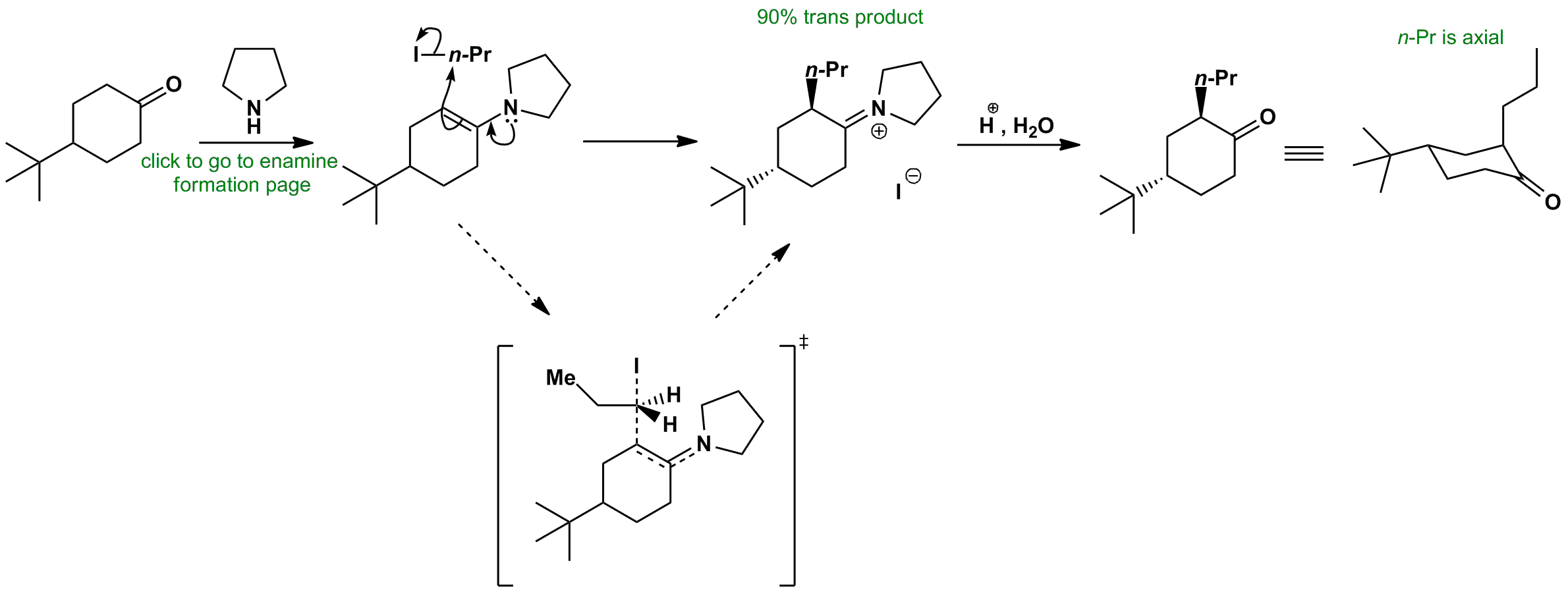
The electrophile (n-PrI) must attack from an axial position in order to interact with the pi orbital of the C=C bond. If it attacks from the same face as the t-Bu group, it will form a twist boat conformation which is unstable. If it attacks from the opposite face, it can form a chair where the t-Bu remains equatorial (stable) and the n-Pr is axial. The chair-like transition state is the most stable option.
F. Johnson, Chem. Rev., 1968, 68, 375–413.




