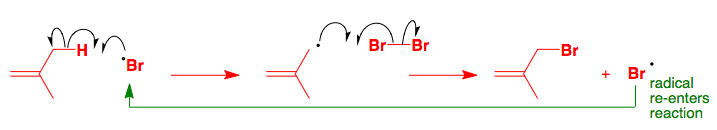
Removal of an alkene hydrogen gives a carbon-centred radical localized on the sp2 atom but the removal of a hydrogen from an allylic methyl group gives a much more stable delocalized allylic radical.
The allylic radical collects a bromine atom from a bromine molecule and produces a new bromine radical that can start a new series of reactions. Like the addition of HBr ,the reaction is a radical chain reaction, and only a small amount of Br2 needs to break down to Br• to initiate the reaction.
I. Saikia, A. J. Borah and P. Phukan, Chem. Rev., 2016, 116, 6837–7042.