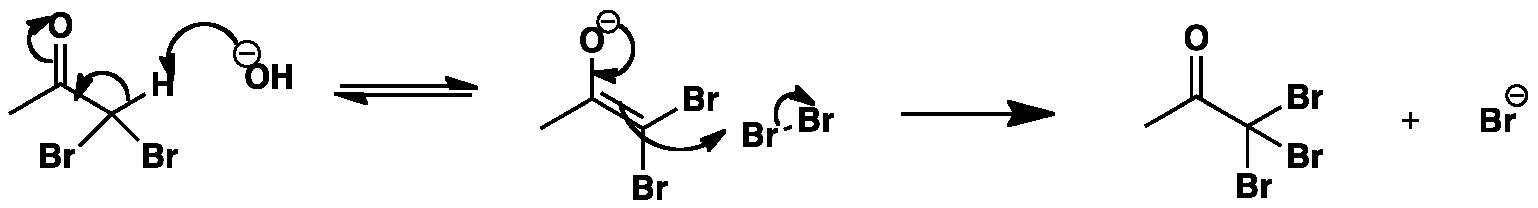
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The reaction is started by the removal of a proton by hydroxide to yield an enolate. The enolate then attacks the bromine molecule to give the tri-substituted bromoacetone and the bromide anion.
On to Next Step | Back to Summary
E. Tapuhi and W. P. Jencks, J. Am. Chem. Soc., 1982, 104, 5758–5765.