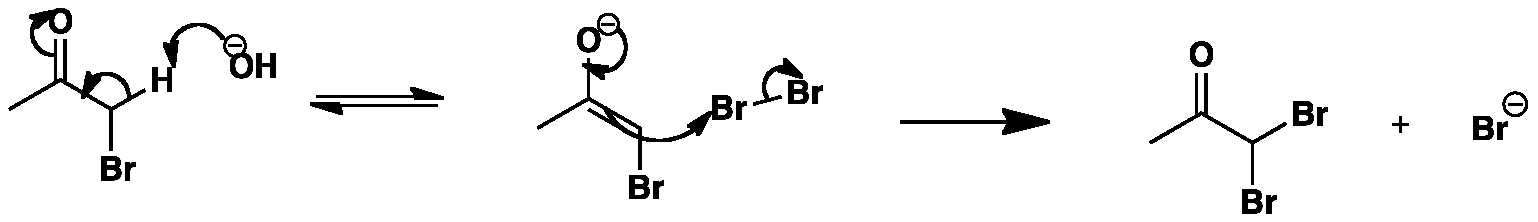
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The presence of the bromine further acidifies the enolisable proton so deprotonation occurs easily to yield an enolate. The enolate then attacks the bromine molecule as before to give the di-substituted bromoacetone and the bromide anion.
On to Next Step | Back to Summary
E. Tapuhi and W. P. Jencks, J. Am. Chem. Soc., 1982, 104, 5758–5765.