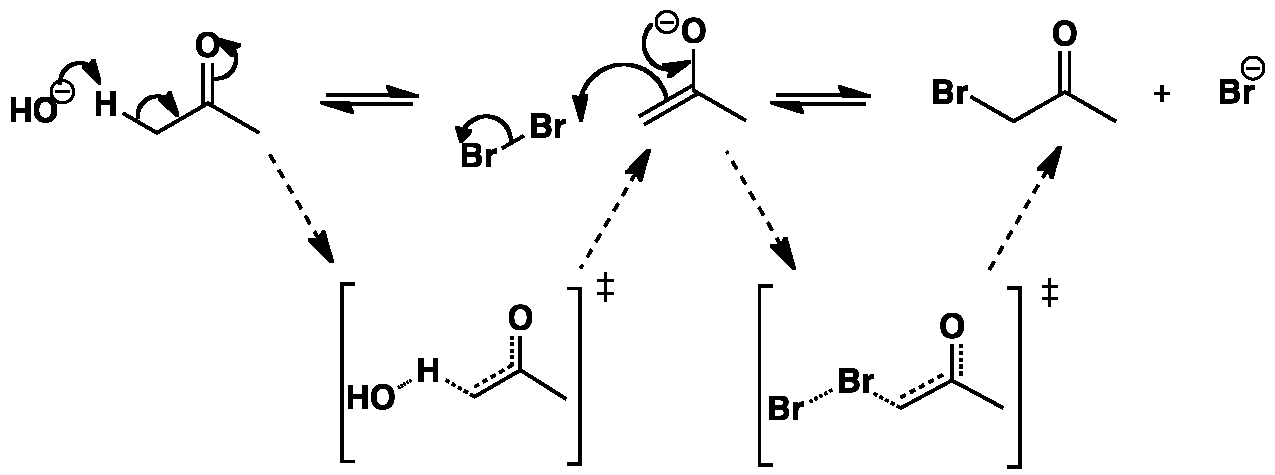
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The proton is removed by the hydroxyl group to yield an enolate. The enolate then attacks the bromine molecule to give bromoacetone and the bromide anion.
On to Next Step | Back to Summary
E. Tapuhi and W. P. Jencks, J. Am. Chem. Soc., 1982, 104, 5758–5765.