Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
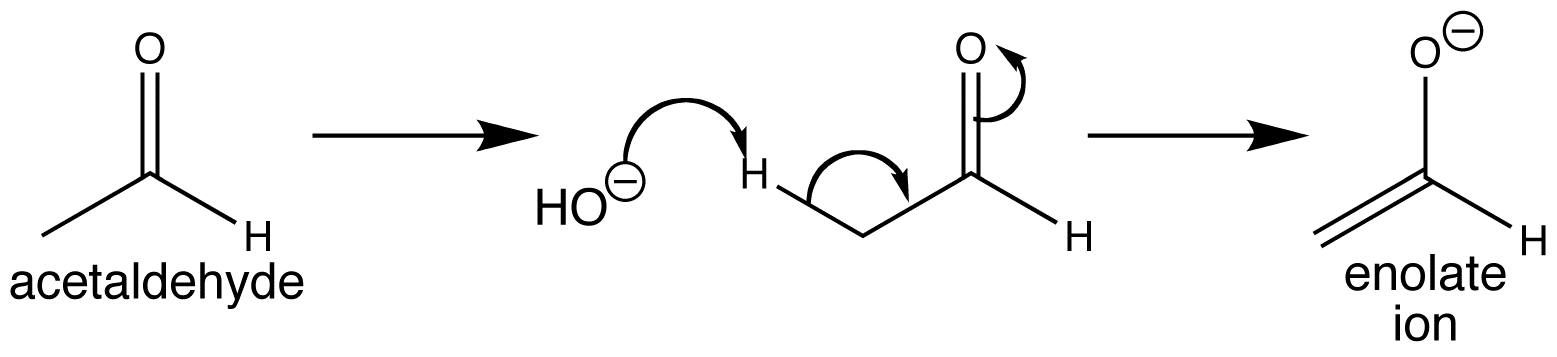
The proton that is removed by the base is perpendicular to the plane of the carbonyl group so that the breaking σ-bond is lined up with the π-bond.
The same mechanism applies to ketones, acids, esters, amides, nitriles etc.
Back to Conjugate addition summary
D. Seebach, Angew. Chemie Int. Ed. English, 1988, 27, 1624–1654.