Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
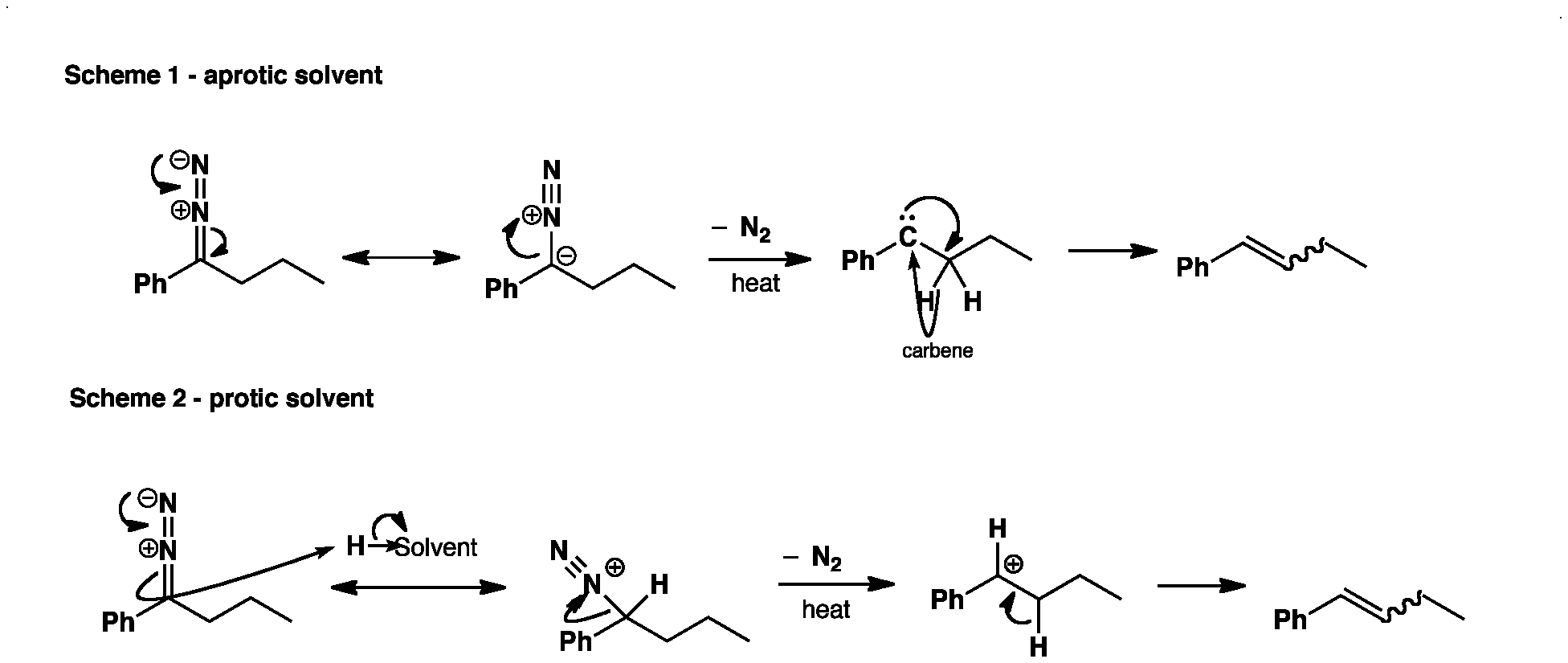
Upon heating the diazo compound it decomposes to give the alkene and N2. In aprotic solvents the decomposition goes via a carbene (Scheme 1) giving predominantly Z-alkenes, whereas in protic solvents the decomposition goes via a carbocation giving a mixture of Z- and E-alkenes (Scheme 2).