
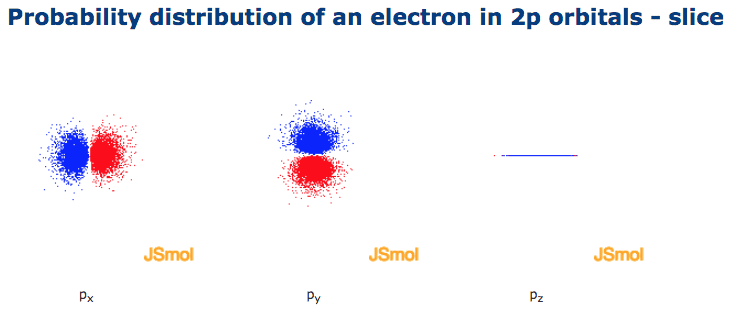
Click the images to see the various views. Nodal planes, where there is no electron density, are displayed after a short delay.
The p sub shell can hold a maximum of six electrons as there are three orbitals within this sub shell. The three p orbitals are at right angles to each other and have a lobed shape. The size of the p orbitals also increases as the energy level or shell increases.
Explore other atomic orbitals
s-orbitals |2p-orbitals |3p-orbitals | 3d-orbitals | 4f-orbitals | Comparison of 1s, 2s and 2p-orbitals