Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
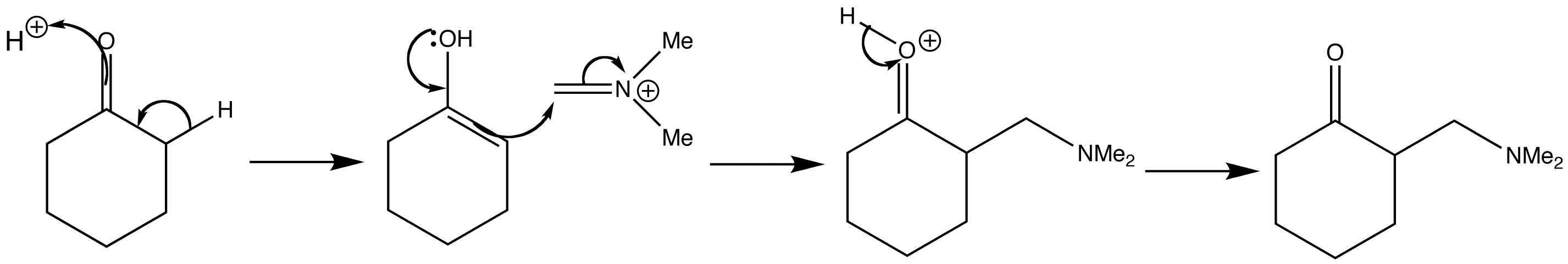
Following tautomerism of cyclohexanone to its enol form, the imine salt can then add to the enol of the ketone in acid solution. The product of the reaction is an amine sometimes called a Mannich base.
T. F. Cummings and J. R. Shelton, J. Org. Chem, 1960, 25, 419–423.
H. G. O. Alvim, E. N. da Silva Júnior and B. A. D. Neto, RSC Adv., 2014, 4, 54282–54299.