Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
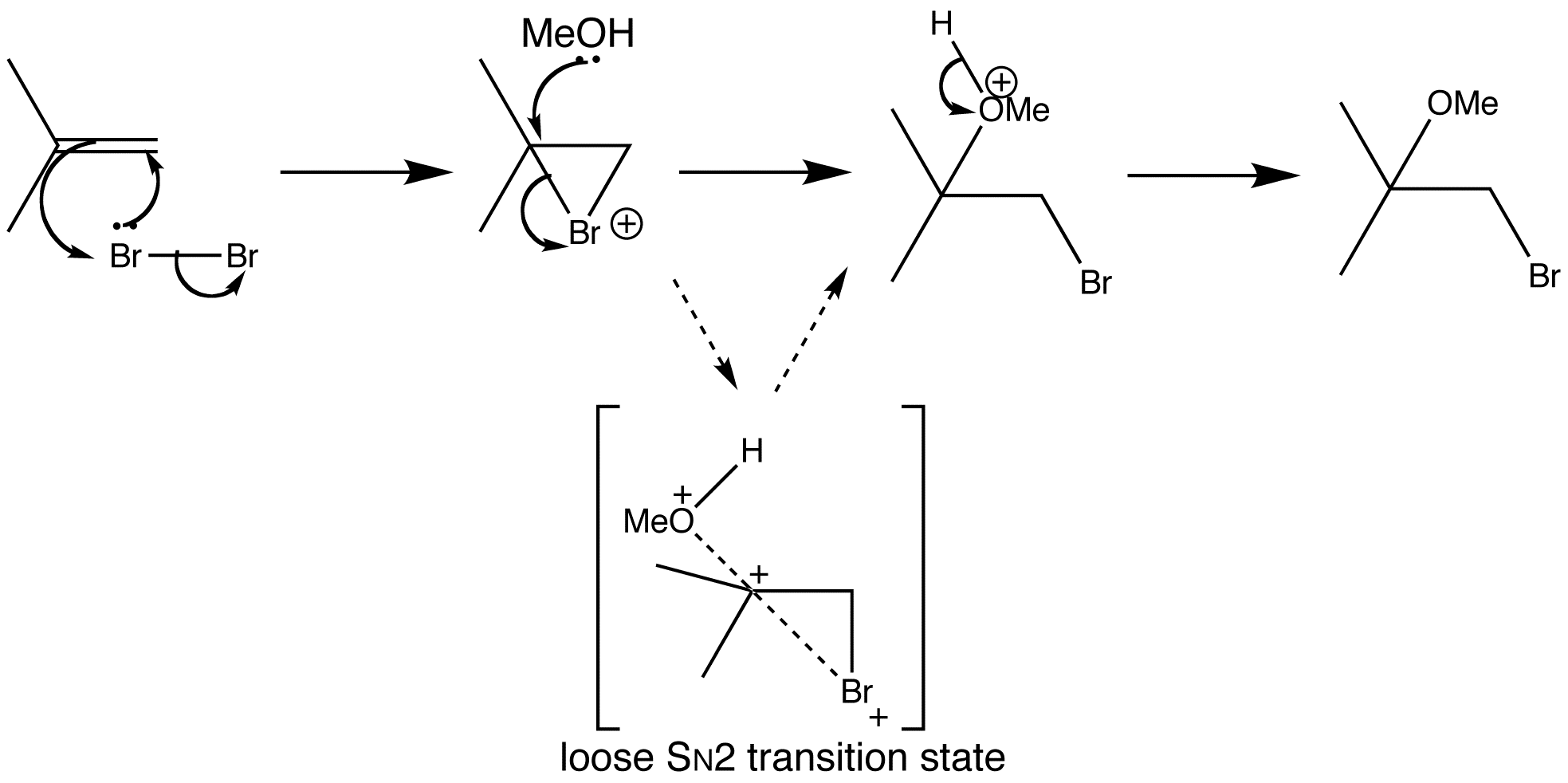
When a bromination reaction is done in a nucleophilic solvent, such as water or methanol, the solvent molecules compete with the bromide to open the bromonium ion. When isobutene is treated with bromine in methanol, an ether is formed by attack of methanol only at the more substituted end of the bromonium ion. Methanol is attacking the bromonium ion where it is most hindered, so there must be some effect at work more powerful than steric hindrance.
Substitution reactions don’t always go by pure SN1 or pure SN2 mechanisms, sometimes the mechanism is somewhere in between. The bromine begins to leave and a partial positive charge builds up at carbon. The departure of bromine can get to a more advanced state at the tertiary end than at the primary end, because the substituents stabilize the build-up of positive charge. This basically suggests that one C-Br bond is longer than the other. Methanol will therefore attack the more charged end with the weaker C-Br bond, giving a ‘loose SN2 transition state’.
J.-E. Dubois and J. R. Chretien, J. Am. Chem. Soc., 1978, 100, 3506–3513.