Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
The phenyl group has been replaced with a methyl group for simplicity.
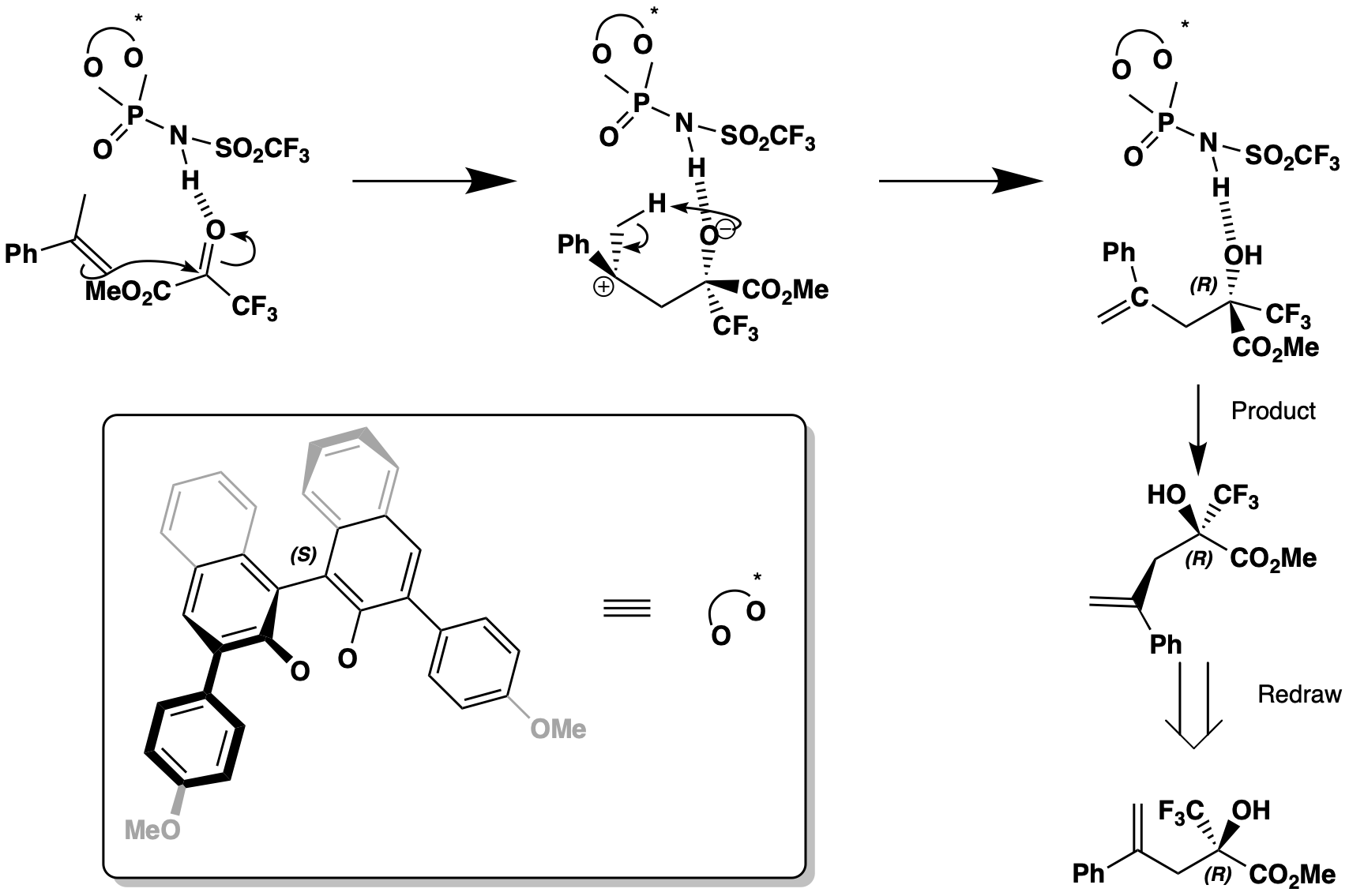
This is the reaction of an alkene with a carbonyl and a chiral phosphate catalyst. This enantioselective reaction only produces the R enantiomer, as the reactants are held in place by interactions from the large chiral phosphate. The alkene attacks the carbonyl group which is more electrophilic due to the electron drawing ester and CF3 groups to form a C-C bond. An allyl tertiary cation is formed, which on work up reforms the double bond to give the R enantiomer.