Go back to Synthesis of Chloroalkanes overview
Click the structures and reaction black arrows in sequence to view the 3D models and animations respectively
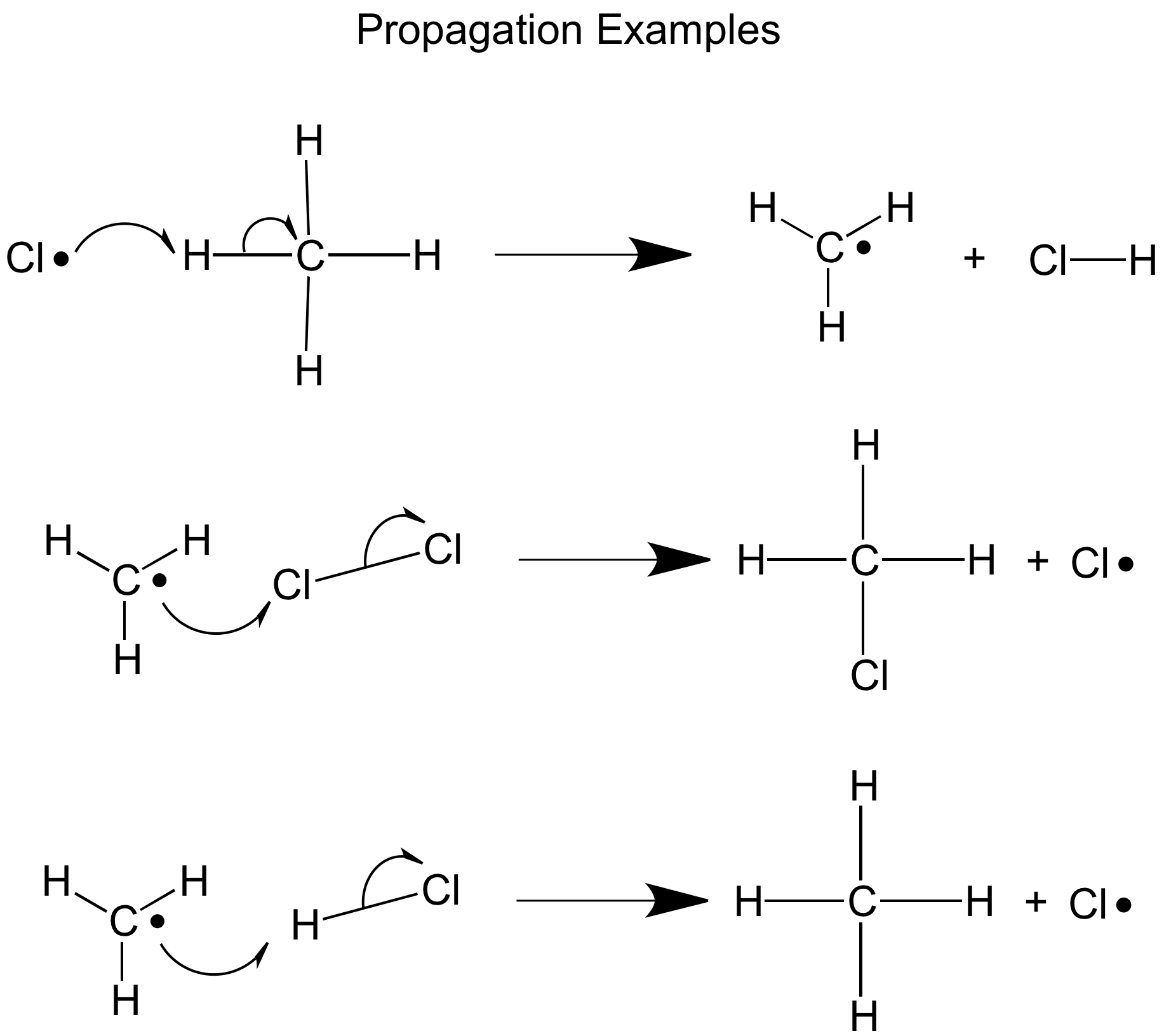
The second step is propagation, in this step free radical species react with neutral molecules creating new free radicals in a chain reaction.
If the chlorine radicals are in excess the hydrogen atoms will eventually all be replaced making CCl4, if the methane atoms are in excess the chlorine will be used up quickly giving CH3Cl as the main product- this is the mostly the case, but it could be worth remembering this for your exam.