Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
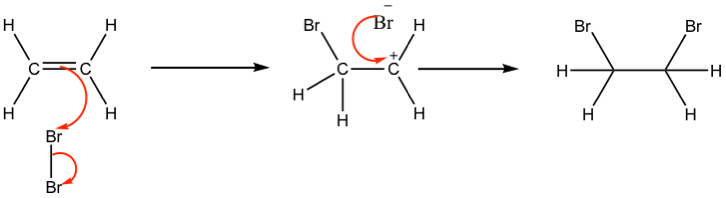
This reaction involves addition of bromine across the double bond. The electron rich part of the double bond causes a dipole to form meaning that bromine becomes electron deficient and therefore becomes the electrophile (hence electrophillic addition). The double bond attacks the bromine and forms a new carbon – bromine bond, while the other carbon atom becomes electron deficient. The resulting carbocation reacts with the bromine ion left from the first step to form the second carbon – bromine bond.
While this mechanism is sufficient at A Level, for those looking to further study in chemistry or are interested, the more correct mechanism (via a bromonium ion) can be found here.