PF5 Phosphorus Pentafluoride
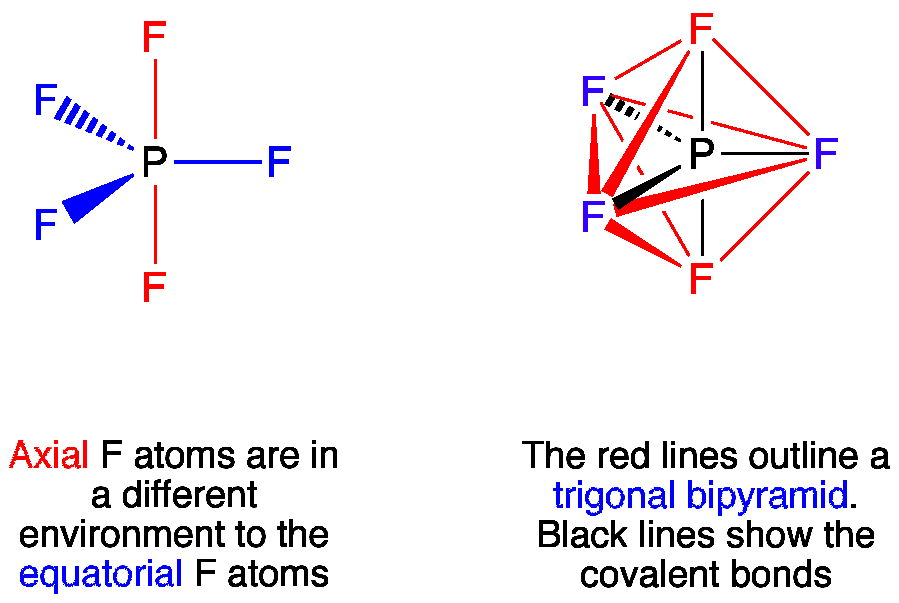
Phosphorus pentafluoride has 5 regions of electron density around the central phosphorus atom (5 bonds, no lone pairs). The resulting shape is a trigonal bipyramidal in which three fluorine atoms occupy equatorial and two occupy axial positions.
The F-P-F angle between equatorial positions is 120°, between the axial and equatorial positions it is 90°.
Click the structures to load the molecules
Related structures H2O | NH3 | CH4 | PF5 |SF4 |ClF3 | SF6 | XeF4




